Photoinduced Fe-catalyzed bromination and iodination of unstrained cyclic alcohols†
Abstract
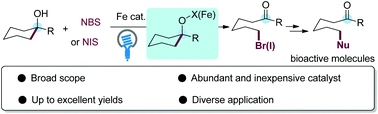
We report a photoinduced iron catalysis for the efficient C–C bond cleavage and bromination or iodination of unstrained tertiary cycloalkanols with NBS/NIS. The reaction features good functional group tolerance and high yields under mild conditions and provides a powerful tool for the preparation of remote halogenated alkyl ketones. The products can be converted via nucleophilic substitution or cross-coupling to other valuable molecules, such as haloperidol, fluanisone, and azabuperone, demonstrating the synthetic value of this reaction.


 Please wait while we load your content...
Please wait while we load your content...