Thermo-induced decarboxylative α-C(sp3)–H fluoroalkylation of glycine derivatives with fluorinated peroxy esters†
Abstract
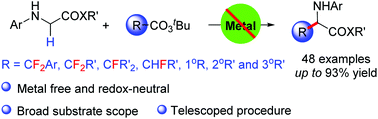
A thermo-induced decarboxylative α-C(sp3)–H fluoroalkylation of glycine derivatives with fluorinated peroxy esters was described. This protocol features transition metal free and redox-neutral conditions, broad substrate scope and excellent functional group tolerance, offering a straightforward route to the fluorinated α-amino acid derivatives in good to excellent yields. Additionally, this strategy was also applicable to the non-fluorinated α-amino acids synthesis.


 Please wait while we load your content...
Please wait while we load your content...