Ni and Fe catalyzed cascade radical reactions of oxime esters with diselenides†
Abstract
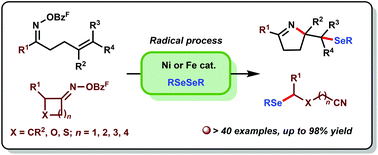
A radical cyclization and ring-opening of oxime esters with diselenides was developed. Both Ni(0) and Fe(II) catalysts could be employed for the selenylation of olefin-containing and cyclic oxime ester derivatives. With this method, a variety of functionalized pyrrolines and alkyl nitriles were synthesized in good yields. Moreover, a mechanism involving iminyl radical and carbon-centered radical intermediates was proposed.


 Please wait while we load your content...
Please wait while we load your content...