Arylations with nitroarenes for one-pot syntheses of triaryl-methanols and tetraarylmethanes†
Abstract
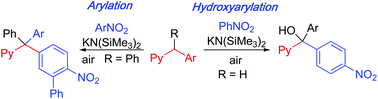
Triarylmethanols are well-known core structures in natural products and pharmacologically relevant compounds. In general, transition metal-based catalysts or highly reactive organometallics are employed for the synthesis of these compounds. Herein, we report the regioselective tandem C(sp3)–H arylation/oxidation of diarylmethanes with nitroarenes to generate arylated alcohols. The present method is general, mild, green, and conducted in air at room temperature. Furthermore, use of triarylmethanes as pro-nucleophiles provides straightforward access to select tetraarylmethanes through a cross-dehydrogenative coupling process.


 Please wait while we load your content...
Please wait while we load your content...
