Photoredox/persistent radical cation dual catalysis for alkoxy radical generation from alcohols†
Abstract
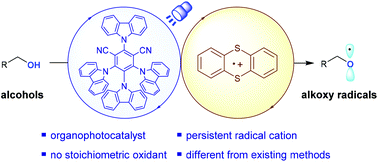
In this report, we present a mild and general strategy for the direct generation of alkoxy radicals from simple aliphatic alcohols enabled by visible-light-induced photoredox/persistent radical cation dual catalysis. The dual catalytic system can trigger alkoxy radical-mediated redox-neutral transformations, avoiding any metal catalysts and the requirement of proximal electron-rich arene moieties on the alcohol substrates. By employing this synergistic catalysis, the Giese reactions of diverse alcohols with electron-deficient alkenes via selective C–C bond scission of the corresponding alkoxy radical intermediates have been achieved. Different from the existing PCET and LMCT processes, mechanistic studies suggest the intermolecular reaction of thianthrene radical cations with alcohols and the subsequent single-electron reduction induced S–O bond homolysis of the formed 5-alkoxythianthrenium cations as the key steps to furnish alkoxy radicals.


 Please wait while we load your content...
Please wait while we load your content...