Site-selective rhodium carbene transfer of 2 hydroxy-β-nitrostyrenes with diazo compounds En route to 2-alkylated benzofurans†
Abstract
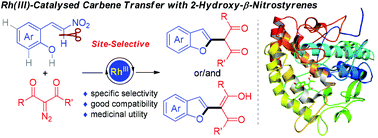
Described herein is a unique rhodium-catalysed site-selective carbene transfer of diazocarbonyl compounds with 2-hydroxy-β-nitrostyrenes en route to 2-alkylated benzofurans. This protocol features simple operation, mild reaction conditions and good functional group tolerance. Mechanistically, the reaction path is proposed to involve a tandem OH-directed vinylic C–H metalation/carbene insertion/protonolysis sequence terminated by Michael addition and nitro elimination. Preliminary bioactivity evaluation reveals that these obtained benzofuran derivatives can act as potential tyrosinase inhibitors, which further strenghtens the utility of the developed transformation.


 Please wait while we load your content...
Please wait while we load your content...