Abstract
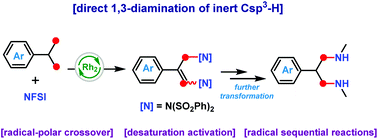
The simultaneous and direct amination of multiple inert Csp3–H bonds is a challenging method for C–N bond formation. Here, we developed a radical sequential reaction mediated by a dirhodium(II) catalyst for the successful one-step synthesis of 1,3-diamines via the interaction of cumene derivatives with N-fluorobenzenesulfonimide (NFSI). Mechanistic studies revealed that the reaction underwent an iterative radical polar crossover and desaturation activation procedure to achieve the activation of three adjacent C–H bonds. The synthesized diamine compounds were converted into useful functional molecules by further removal of phenylsulfonyl groups.


 Please wait while we load your content...
Please wait while we load your content...