Decarboxylative amination of benzoic acids bearing electron-donating substituents and nonactivated amines†
Abstract
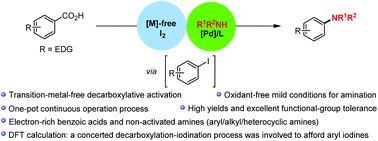
Efficient methods for decarboxylative activation of benzoic acids into great valuable products are highly sought after. Here we report a highly desirable and straightforward decarboxylative amination of readily available benzoic acids, with the long-inaccessible extension of decarboxylative amination to normally poorly reactive electron-rich benzoic acids and non-activated amines in moderate to high yields with excellent functional-group tolerance. This aniline synthesis is applicable to a wide range of aryl/alkyl/heterocyclic amines under oxidant-free conditions, preventing adverse reactions compared to traditional methods. It also provides decarboxylative C–N modifications of complex bioactive nitrogen-bearing molecules to highlight the synthetic utility of this protocol. The theoretical calculation has supported that this decarboxylative amination may proceed via a concerted decarboxylation–iodination-type process to afford aryl iodine intermediates, which promoted a palladium-catalyzed C–N cross-coupling pathway under mild conditions. Notably, this efficient reaction features a fine choice of the same base/solvent system to successfully achieve two independent reactions in a one-pot continuous operation process. This strategy provides a method for previously inaccessible decarboxylative amination of benzoic acids bearing electron-donating substituents, as a new alternative to the existing decarboxylative oxidative couplings.


 Please wait while we load your content...
Please wait while we load your content...