Facile construction of the all-bridge-position-functionalized 2,4,6,8-tetraazaadamantane skeleton and conversion of its N-functionalities†
Abstract
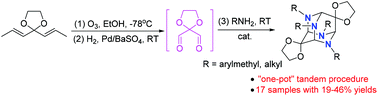
The 2,4,6,8-tetraazaadamantane skeleton is a novel rigid symmetric cage-like organic structure and the construction of this kind of scaffold is a long-standing challenge. In this article, an unusual protocol of a “one-pot” three-step strategy to build the special all-bridge-position-functionalized tetraazaadamantane skeletons from 2,2-dipropenyl-1,3-dioxolane via ozonation, hydrogenation with 5% Pd/BaSO4, and condensation of the in situ generated dial intermediate with primary amines is reported for the first time. Different primary amines are compatible with this process, and the corresponding 2,4,6,8-tetraalkyl-2,4,6,8-tetraazaadamantane-9,10-dione bis(ethylene ketals) were achieved in 19–46% yields. The conversion of the N-benzyl group to some other functionalities such as acetyl, benzoyl, nitroso and nitro was also investigated.


 Please wait while we load your content...
Please wait while we load your content...