Reaction mechanism study on reactions of phenylacetylenes with HSnEt3 promoted by B(C6F5)3 with and without DABCO†
Abstract
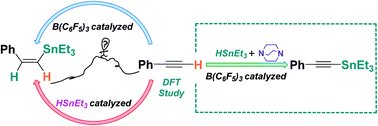
There are many methods used to transform phenylacetylenes. Most efforts have been focused on dehydrogenative coupling, although nucleophilic addition is often observed as a side reaction. The selectivity of these reactions used to be controlled by transition metal complexes as catalysts or bases as additives. Dehydrogenative silylation of phenylacetylenes has been achieved in satisfactory yield by metal-free B(C6F5)3/DABCO. Although alkynylstannanes generated by dehydrogenative stannylation can be selectively generated by transition metals, vinylstannanes remain as the major products when catalyzed by metal-free B(C6F5)3. Based on this, a mechanism study on B(C6F5)3-catalyzed hydrostannation and dehydrogenative stannylation of phenylacetylene was carried out in this work. The density functional theory calculations show that the B(C6F5)3-promoted hydrostannation reaction is kinetically facile without DABCO, but the major reaction that occurs in the presence of DABCO is dehydrogenative stannylation.


 Please wait while we load your content...
Please wait while we load your content...