Sonogashira cross-coupling as a key step in the synthesis of new glycoporphyrins†
Abstract
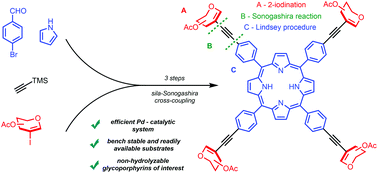
Glycoporphyrins are an important subclass of the porphyrin family of compounds. They have been intensively studied because of their biological importance, i.e. they exhibit increased solubility in polar solvents and they might provide better selectivity towards biological targets. New methods for the efficient synthesis of these compounds are highly desired. Unfortunately, in most of the described methodologies, carbohydrate-linked porphyrins are obtained through hydrolyzable connections. Herein, the original route to new C–C conjugated glycoporphyrins via the Sonogashira cross-coupling is presented. The optimal conditions have been established and the most convenient synthetic strategy has been determined.


 Please wait while we load your content...
Please wait while we load your content...