Room temperature synthesis of perylene diimides facilitated by high amic acid solubility†
Abstract
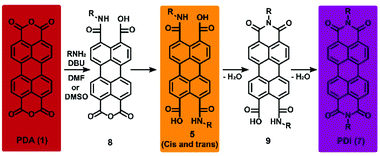
A novel protocol for the synthesis of perylene diimides (PDIs), by reacting perylene dianhydride (PDA) with aliphatic amines is reported. Full conversions were obtained at temperatures between 20 and 60 °C, using DBU as the base in DMF or DMSO. A “green” synthesis of PDIs, that runs at higher temperatures, was developed using K2CO3 in DMSO. The reaction sequence for the imidization process, via perylene amic acid intermediates (PAAs), has been confirmed experimentally aided by the synthesis and full characterization of stable model amic acid salts and amic esters. Kinetic studies, using absorption spectroscopy, have established that PDI formation proceeds via fast amic acid formation, followed by a slow conversion to imides. Solubility of the intermediate PAA salts is found to be low and rate-limiting. Based on this finding, quantitative PDI synthesis at room temperature was achieved by diluting the reaction mixture with water, the solvent in which PAA salts have better solubility. Thus, the otherwise harsh synthesis of PDIs has been transformed into an extremely convenient functional group tolerant and highly efficient reaction that runs at room temperature.


 Please wait while we load your content...
Please wait while we load your content...