Silver-catalyzed stereoselective C-4 arylthiodifluoromethylation of coumarin-3-carboxylic acids via a double decarboxylative strategy†
Abstract
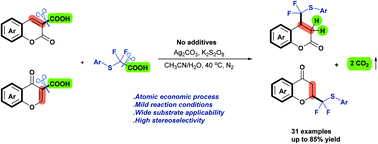
A facile silver-catalyzed dual decarboxylation of arylthio-difluoroacetic acid with coumarin-3-carboxylic acids/chromone-3-carboxylic acids was developed. This method provided a unique way to synthesize a series of C-4 arylthiodifluoromethylated 3,4-dihydrcoumarins/C-2 arylthiodifluoro-methylated chromanone derivatives in moderate to good yields under mild conditions. Mechanistic studies confirmed that the methodology proceeds via a bimolecular decarboxylative radical pathway. In addition, experimental studies showed that the C-3 carboxyl group of coumarin-3-carboxylic acids/chromone-3-carboxylic acids and the CF2 group of arylthiodifluoroacetic acids play a crucial role in this transformation.


 Please wait while we load your content...
Please wait while we load your content...