1D alignment of proteins and other nanoparticles by using reversible covalent bonds on cyclic peptide nanotubes†
Abstract
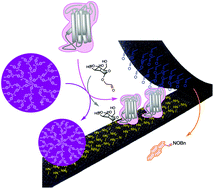
Self-assembling cyclic peptide nanotubes are supramolecular structures whose diameter and external surface properties are precisely controlled. In this communication we describe a general strategy to align different molecules on top of the nanotube surface by using cyclic peptides bearing chemoreversible reactive groups. D,L-α-Cyclic peptides endowed with a hydrazide moiety were condensed with a pyrenecarboxyaldehyde to facilitate the nanotube formation and deposition on flat unfunctionalized surfaces. The hydrazide moieties can be liberated without affecting the tubular structure and then used to align on top of the nanotube other molecules bearing aldehyde functions. Additionally, the attachment of specific ligands allowed the supramolecular alignment of corresponding receptors, such as mannosyl aldehyde and concavaline-A, driven by the nanotube structure through complemetary protein–ligand interactions.


 Please wait while we load your content...
Please wait while we load your content...