Dispiroacridine-indacenobisthiophene positional isomers: impact of the bridge on the physicochemical properties†
Abstract
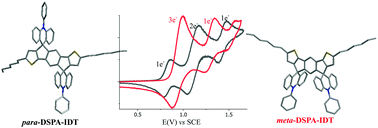
We report the influence of positional isomerism on the electronic (electrochemical HOMO/LUMO energy levels), photophysical and physical properties (molecular organization, crystallinity and phase transitions) and charge transport properties of dispiroacridine-indacenobisthiophene positional isomers. The isomers differ from the central indacenobisthiophene (IDT) core, which displays either para or meta linkages. We show that the spiro-connected phenylacridine bridges have a significant influence on all these properties and particularly on the charge transport mobility values, which were found to be higher in the meta isomer than in the para isomer. This finding is different to what was reported in the literature for the other couples of IDT-based isomers and shows the key role played by the spiro-connected fragments in these molecular systems.


 Please wait while we load your content...
Please wait while we load your content...