Ad aurum: tunable transfer of N-heterocyclic carbene complexes to gold surfaces†
Abstract
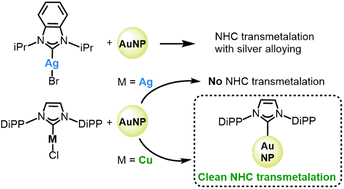
The exceptional stability of N-heterocyclic carbene (NHC) monolayers on gold surfaces and nanoparticles (AuNPs) is enabling new and diverse applications from catalysis to biomedicine. Our understanding of NHC reactivity at surfaces; however, is quite nascent when compared to the long and rich history of NHC ligands in organometallic chemistry. In this work, well-established transmetalation reactions, previously developed for NHC transfer in homogeneous organometallic systems, are explored to determine how they can be used to create carbene functionalized gold surfaces. Two classes of NHCs, based on imidazole and benzimidazole scaffolds, were tested. The resulting AuNP surfaces were analyzed using X-ray photoelectron spectroscopy (XPS), laser desorption ionization mass spectrometry (LDI-MS), and surface-enhanced Raman spectroscopy (SERS). Reaction of either a Au(I) or Ag(I) isopropyl benzimidazole NHC complex with citrate-capped AuNPs yields, in both cases, a chemisorbed NHC that is bound through a Au adatom. Theoretical calculations additionally illustrate that binding through the Au adatom is favored by more than 10 kcal mol−1, in good agreement with experiments. Surprisingly, reaction of Au(I), Ag(I), and Cu(I) diisopropylphenyl imidazole NHCs do not follow the same pattern. The Cu complex undergoes transmetalation with very little deposition of Cu; whereas, unexpectedly, the Ag complex foregoes transmetalation and instead adducts to the AuNP with retention of the Ag–C bond. Theoretical calculations illustrate that the imidazole ligand affords significant dispersion interactions with the gold surface, which may stabilize binding through the Ag adatom motif, despite its less favorable bonding energies. Taken together these results suggest a unique ability to tune the reactivity by changing the carbene structure and raise critical questions about how established transmetalation reactions in organometallic chemistry can be applied to form NHC functionalized surfaces.


 Please wait while we load your content...
Please wait while we load your content...