Ferritin nanocomposites for the selective delivery of photosensitizing ruthenium-polypyridyl compounds to cancer cells†
Abstract
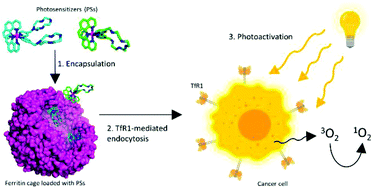
Herein, we report on the successful encapsulation into human H-ferritin of two highly charged Ru(II) polypyridyl complexes, [Ru(phen)2L′]2+ (Ru1, with L′ = 4,4′-(2,5,8,11,14-pentaaza[15])-2,2′-bipyridilophane) and [Ru(phen)2L′′]2+ (Ru2, with L′′ = 4,4′-bis-[methylen-(1,4,7,10-tetraazacyclododecane)]-2,2′-bipyridine). The resulting Ru(II)-ferritin nanocomposites are highly luminescent, display great stability in physiological conditions and preserve the native shell–core structure of the protein. Moreover, the singlet oxygen sensitizing properties of metal complexes, established by independent spectrophotometric and spectrofluorimetric measurements, are largely maintained also in their encapsulated form. Ru(II)-ferritin nanocomposites are exclusively internalized by cancer cells expressing the transferrin receptor 1 (TfR1) (i.e. HeLa and A2780 with respect to non-cancerous C2C12 myoblasts). Immunofluorescence analysis also reveals the co-localization of Ru1 and Ru2 with the TfR1 in the internal cellular compartments of HeLa and A2780 cells, highlighting the crucial role played by the receptor-mediated endocytosis of H-type ferritins exerted by TfR1. Finally, an MTT assay probed that light-activation effectively leads to a marked dose-dependent cytotoxic effect uniquely against cancer cells. This study underlines the potential of human H-ferritin as a valuable tool for the tumor-targeted delivery of sensitizing agents for photodynamic therapy.


 Please wait while we load your content...
Please wait while we load your content...