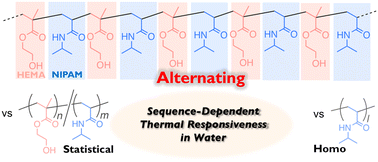
Precision syntheses of poly(NIPAM-alt-HEMA) and effects of the alternating sequence on thermoresponsive behaviors in water†
Abstract
In this work, an alternating copolymer of 2-hydroxyethyl methacrylate (HEMA) and N-isopropylacrylamide (NIPAM) [poly(NIPAM-alt-HEMA)] was synthesized via ATRP-based cyclopolymerization of a divinyl monomer carrying a transformable linker and aminolysis transformation of the resulting cyclopolymer with isopropylamine. The selective propagation in the cyclopolymerization leading to the alternating sequence was supported by the reactivity ratios of the two model monomers cut out from the divinyl monomer. The quantitative transformation and the alternating sequence were confirmed by 1H NMR, FT-IR, and MALDI-TOF-MS. The thus-obtained poly(NIPAM-alt-HEMA) underwent an LCST-type temperature-induced phase transition in water, and the thermoresponsive behavior was different from that of the corresponding statistical copolymers [poly(NIPAM-sta-HEMA)] as well as the homopolymer of NIPAM. The thermoresponsiveness specific to the alternating sequence is likely derived from the structural character that can affect the dehydration behavior, such as no existence of the consecutive NIPAM sequence and the adjacent circumstance of NIPAM units and hydroxy pendants of HEMA.


 Please wait while we load your content...
Please wait while we load your content...