Diverse chemically recyclable polymers obtained by cationic vinyl and ring-opening polymerizations of the cyclic ketene acetal ester “dehydroaspirin”†
Abstract
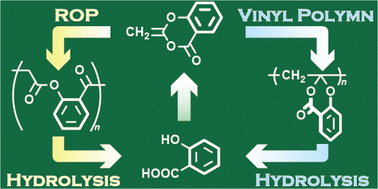
In the quest for sustainable polymers, preparing two different types of recyclable polymer from only one monomer is efficient and interesting. Herein, we report the polymerizations of 2-methylene-4H-benzo[d][1,3]dioxin-4-one (MBDO or dehydroaspirin) to afford degradable polymers by two reaction modes, vinyl polymerization (VP) and ring-opening polymerization (ROP). The radical polymerization proceeded in VP selectively, and the monomer reactivity was evaluated as Q = 0.13 and e = −1.14, by copolymerization with methyl methacrylate. The product of VP was found to be degradable by acid and base hydrolysis to afford acetic acid and salicylic acid, that is, the raw materials of MBDO. The e value suggested that MBDO is cationically polymerizable. In fact, cationic polymerization resulted in both VP and ROP; in particular, polymerization in the presence of EtAlCl2 below 0 °C proceeded in selective ROP to afford a polyester composed of a β-ketoester unit that exhibited degradability by basic hydrolysis to the raw materials of MBDO. Therefore, MBDO is a chemically recyclable monomer to afford diverse sustainable polymers by VP and ROP.
- This article is part of the themed collection: Plastic Conversion


 Please wait while we load your content...
Please wait while we load your content...