Nickel-catalysed cycloaddition oligomerisation of 1,6-diynes to medium-size cyclic polyenes†
Abstract
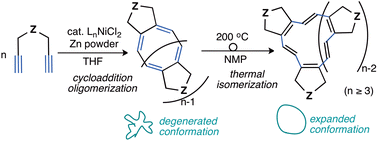
Nickel complexes having a sterically demanding N,N- or N,N,N-type ligand are demonstrated to catalyse the cycloaddition oligomerisation of 1,6-heptadiynes to produce medium-size cyclic polyenes selectively with narrow molecular dispersity and minimum side reactions. The resulting cyclic polyenes exhibit non-conjugated behaviour on NMR and UV–Vis. absorption analyses. Meanwhile, regio-isomerised cyclic polyenes including trans-disubstituted alkenes, which are generated via thermal isomerisation of the cyclic polyenes at 200 °C, exhibit conjugated behaviour. The present oligomerisation reaction has wide functional group compatibility, enabling the production of medium-size cyclic polyenes bearing various functional groups. A plausible reaction mechanism that accounts for the narrow polydispersity is proposed.


 Please wait while we load your content...
Please wait while we load your content...