Geometric requirements for living anionic polymerization: polymerization of rotationally constrained 1,3-dienes†
Abstract
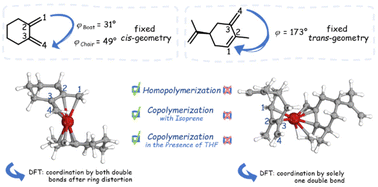
The living anionic copolymerization of 1,3-dienes such as isoprene (I) or butadiene (B) can afford a variety of different polymer microstructures which determine the material's properties. One decisive factor for the stereochemistry is the polarity of the medium, which can be ascribed to the proposed coordinative mechanism in nonpolar media. In a combined synthetic, kinetic and theoretical study the homopolymerization of two new 1,3-diene monomers with a rigid, prescribed cisoid or transoid geometry as well as their copolymerization with isoprene is examined in cyclohexane as a typical nonpolar solvent. Real-time 1H NMR spectroscopy provides kinetic data and hence the reactivity ratios at different temperatures. Furthermore, reaction pathways are modelled and followed by density functional theory (DFT) calculations to support the experimental observations. The results show that a cisoid geometry with in-plane double bonds is mandatory for propagation, and that the necessary ring distortion in the case of the cyclic cisoid diene accounts for its higher activation barrier, leading to gradient formation in statistical copolymerization experiments with isoprene.


 Please wait while we load your content...
Please wait while we load your content...