Facile synthesis of eight-membered cyclic(ester-amide)s and their organocatalytic ring-opening polymerizations†
Abstract
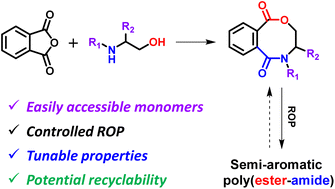
Five eight-membered cyclic(ester-amide)s (M1–M5) were synthesized from phthalic anhydride and β-amino alcohols by sequential nucleophilic addition and intramolecular esterification. The organocatalytic ring-opening polymerization (ROP) of these monomers with 1,5,7-triazabicyclo[4.4.0]-dec-5-ene (TBD) and 1,8-diazabicyclo[5.4.0]undecane-7-ene (DBU)/thiourea (TU) as the catalysts was investigated, and they could all be polymerized in a controlled manner under optimized conditions, affording well-defined poly(ester-amide)s (PEAs) (P1–P5) with tailored molar masses and narrow dispersities. The structures of these PEAs were characterized, confirming that the main-chain tertiary amide bonds existed as a mixture of cis/trans isomers. These PEAs are amorphous materials with high thermal stability (Td,5%: 260–301 °C) and side chain-dependent glass transition temperatures (Tgs) (62–138 °C). Polymer P1 contains both a rigid benzene ring and a pyrrole ring in the backbone, being a PEA with the highest Td,5% (301 °C) and Tg (138 °C). The TBD-catalyzed copolymerization of M1 and rac-LA could generate a series of random copolymers with tunable and enhanced Tgs (52–96 °C) with increasing incorporation ratio of M1 (0–51 mol%). Finally, These PEAs could be selectively and completely converted into their corresponding monomer precursors in alkaline aqueous solutions.


 Please wait while we load your content...
Please wait while we load your content...