Molecularly engineered dual-crosslinked elastomer vitrimers with superior strength, improved creep resistance, and retained malleability†
Abstract
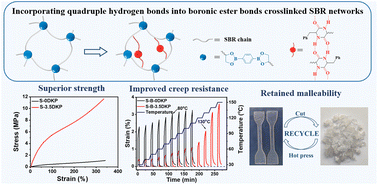
It is very challenging to prepare elastomer vitrimers that are both robust and dimensionally stable in service. To address this issue, we have initiated a multi-phase design of elastomer vitrimers by incorporating quadruple hydrogen bonds (H-bonds) into vitrimer networks. Specifically, commercialized styrene–butadiene rubber (SBR) was functionalized with the synthesized 5-benzyl-3,6-dioxo-2-piperazineacetic acid (DKP) to introduce amide functionalities, which was then crosslinked by dimercapto-borate via thiol–ene “click” chemistry. The H-bonds between amide moieties functioned as sacrificial units, which could undergo reversible breakage and recombination events, and therefore improve the modulus, ultimate strength, and toughness simultaneously. Moreover, with an aromatic ring and a symmetrical amide group in the structure, the grafted DKP could self-assemble via hydrogen bonds, leading to the formation of a microphase-separated structure in the elastomer matrix. Besides, the creep resistance was improved as the H-bonds were physical crosslinks that imposed additional constraints on chain segment diffusion. Nevertheless, the reprocessability of the network was not affected as the H-bonds and boronic ester crosslinks could dissociate and exchange at elevated temperatures, respectively.


 Please wait while we load your content...
Please wait while we load your content...