Taking advantage of β-hydroxy amine enhanced reactivity and functionality for the synthesis of dual covalent adaptable networks†
Abstract
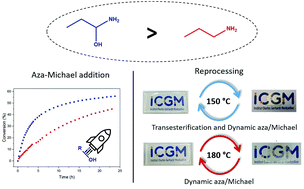
This study demonstrates the advantage of using β-hydroxy amine monomers for the synthesis of CANs. The increased reactivity of β-hydroxy amines towards the aza-Michael addition compared to their alkyl equivalents was highlighted by kinetic analyses coupled with rheological experiments. New catalyst-free covalent adaptable networks (CANs) were thus synthesized by poly aza-Michael addition using either β-hydroxy amine or its non-hydroxy analog. These CANs exhibit dynamic aza-Michael exchange under thermal stimulus. The synergistic effect of exchange reactions was highlighted by stress-relaxation and frequency sweep analyses. CANs were finally reshaped and their chemical and physical properties were compared to the initial ones.


 Please wait while we load your content...
Please wait while we load your content...