Thermally activated delayed fluorescence of carbazole-benzophenone dendrimers with bulky substituents†
Abstract
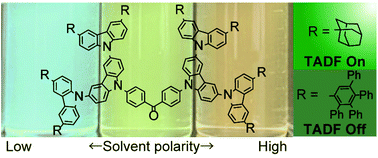
Carbazole dendrimers with a benzophenone core and bulky terminal substituents were synthesized, and their thermally activated delayed fluorescence (TADF) properties were investigated. The adamantane (Ad) substituted dendrimer showed green TADF emission with a PLQY of 40% in the neat film. The tetraphenylphenyl (TPPh) substituted dendrimer showed blue emission with a PLQY of 11% in the neat film but did not show effective TADF. The dendrimer and dendron (fragment) phosphorescence measurement revealed that the TPPh substituted carbazole dendron has low triplet energy. This will reduce the TADF efficiency. Also a new deactivation path due to the large freedom in the rotation of TPPh substituents leads to non-radiative deactivation and quenches the TADF emission. Simple terminal modification can minimize the intermolecular interaction and tune the on–off of the TADF, which gives insights for designing efficient TADF materials.


 Please wait while we load your content...
Please wait while we load your content...
