Effect of halogen and solvent on iron-catalyzed atom transfer radical polymerization†
Abstract
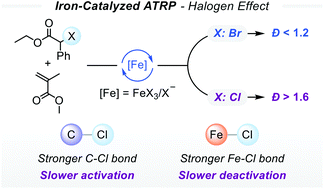
Efficient transfer of halogen atoms is essential for controlling the growth of polymers in atom transfer radical polymerization (ATRP). The nature of halogens may influence the efficiency of the halogen atom transfer during the activation and deactivation processes. The effect of halogens can be associated with the C–X bond dissociation energy and the affinity of the halogens/halides to the transition metal catalyst. In this paper, we study the effect of halogens (Br vs. Cl) and reaction media in iron-catalyzed ATRP in the presence of halide anions as ligands. In Br-based initiating systems, polymerization of methacrylate monomers was well-controlled whereas Cl-based initiating systems provided limited control over the polymerization. The high affinity of the Cl atom to the iron catalyst renders it less efficient for fast deactivation of growing chains, resulting in polymers with molecular weights higher than predetermined by Δ[M]/[RX]o and with high dispersities. Conversely, Br can be exchanged with higher efficiency and hence provided good control over polymerization. Decreasing the polarity of the reaction medium improved the polymerization control. Polymerizations using ppm levels of the iron catalyst in acetonitrile (a more polar solvent) yielded polymers with larger dispersity values due to the slow rate of deactivation as opposed to the less polar solvent anisole, which afforded well-controlled polymers with dispersity <1.2.
- This article is part of the themed collections: Chemistry of polymers - Chemical Science symposium collection and Synthetic Methodologies for Complex Macromolecular Structures


 Please wait while we load your content...
Please wait while we load your content...