Hydrogen bond templated synthesis of catenanes and rotaxanes from a single isophthalic acid derivative†
Abstract
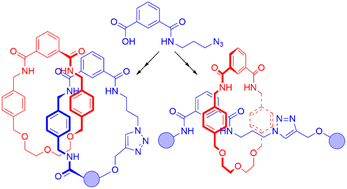
Hydrogen bond templated [2]catenanes and [2]rotaxanes have been synthesized using azide precursors derived from a single isophthalic acid derivative precursor. The interlocked molecules were prepared using either stoichiometric or near stoichiometric amounts of macrocycle and CuAAC “click” precursors, with yields of up to 70% for the mechanical bond formation step. Successful preparation of the interlocked structures was confirmed by NMR spectroscopy and mass spectrometry, with detail of co-conformational behaviour being elucidated by a range of 1H NMR spectroscopic experiments.


 Please wait while we load your content...
Please wait while we load your content...