A new phosphoramidite enables orthogonal double labelling to form combination oligonucleotide probes†
Abstract
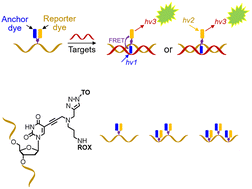
Oligonucleotides labelled with thiazole orange intercalator and a reporter dye on the same thymine base have been synthesized. The key phosphoramidite (AP-C3 dT) contains an alkyne and amine, enabling dual orthogonal labelling of the nucleobase. Multiple monomers can be added to produce heavily functionalised oligonucleotides. In their DNA and 2′-OMe RNA formats these combination probes display high duplex stability and fluorescence when bound to complementary DNA and RNA.


 Please wait while we load your content...
Please wait while we load your content...