Design, synthesis, spectroscopic characterization, computational analysis, and in vitro α-amylase and α-glucosidase evaluation of 3-aminopyridin-2(1H)-one based novel monothiooxamides and 1,3,4-thiadiazoles†
Abstract
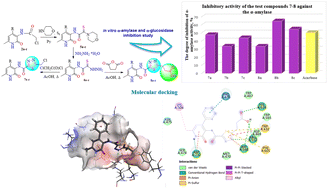
Due to the growth in the incidence of diabetes mellitus throughout the world, the urgency in the search for new safe and effective inhibitors of α-amylase and α-glucosidase is increasing. In this work, we attempted to carry out studies on the synthesis, modification and comprehensive computer and biological research of new derivatives of monothiooxamides. It was shown that monothiooxamides based on 3-aminopyridin-2(1H)-ones enter into transamidation reactions with hydrazine hydrate to form the corresponding thiohydrazides. Furthermore, under the action of chloroacetyl chloride and succinic anhydride, these thiohydrazides are cyclized into conjugated 1,3,4-thiadiazole derivatives. The possible biological activity of the obtained products was evaluated by molecular docking using the AutoDock Vina program. Compounds 7a and 8b showed higher binding affinities for selected target proteins compared to the known anti-diabetic drugs acarbose and TAK-875. The obtained new derivatives of 1,3,4-thiadiazole showed sufficiently high values of inhibitory activity in the in vitro test against the enzymes α-amylase and α-glucosidase as well as sufficiently low IC50 values (for 8b 122.2 μM), which is 8 times less than the value for the reference drug acarbose (998.3 μM). All synthesized derivatives of monothiooxamides 5–8(a–c) showed no cytotoxic properties at physiological concentrations in the MTT test in human neonatal dermal fibroblasts. Moreover, some compounds (6a–c, 7a–c, 8b,c) showed pronounced cytoprotective activity. Thus, the compounds 5–8(a–c) synthesized by us, both according to the results of computer calculations and in vitro biological screening, showed their potential antidiabetic activity and high prospects for further in-depth studies, including in vivo studies.


 Please wait while we load your content...
Please wait while we load your content...