Photoinduced arylation of chloroarenes in flow: synthesis of unsymmetrical biaryls†
Abstract
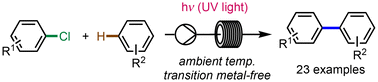
A photoflow method is presented for a radical-based coupling of unactivated arenes and aryl chlorides. The process proceeded smoothly at ambient temperature under metal-free conditions. Of note is that the reaction conditions are fine-tuned for chloroarenes with different electronic properties. While the reactivity profile of aryl chlorides is generally known to be inferior to those of the corresponding iodides and bromides, we demonstrate that the title protocol is efficient in converting readily available and inexpensive chloroarenes into unsymmetrical biaryl products.


 Please wait while we load your content...
Please wait while we load your content...