Computational investigation of cycloadditions between cyclopentadiene and tropone-3,4-dimethylester†
Abstract
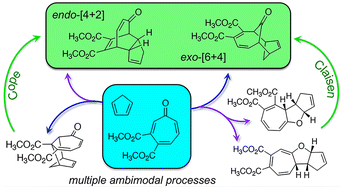
Thermally promoted cycloaddition reactions of tropone-3,4-dimethylester and cyclopentadiene have been investigated using density functional theory calculations at the M06-2X level and the CBS-QB3 method. The reaction shares several characteristics with previously investigated cycloadditions involving unsubstituted tropone and cyclopentadiene, however substitution of the tropone component with methyl esters results in lower transition state free energy barriers, greater thermodynamic driving forces, and a significant increase in the number of possible pericyclic reaction pathways. Eighteen different [4 + 2], [6 + 4], or [8 + 2] cycloaddition products are possible and many of the initially formed cycloadducts can be interconverted through Cope or Claisen rearrangements. Of the many possible cycloaddition products only two are predicted to form: the exo-[6 + 4] product and one [4 + 2] product where the substituted tropone appears to be the diene. The two computationally predicted products are the same as the two that are observed experimentally, however computations indicate that both products result from ambimodal processes rather than single-step (monomodal) cycloaddition pathways.


 Please wait while we load your content...
Please wait while we load your content...