A DFT study of the active role of the phosphate group of an internal aldimine in a transamination reaction†
Abstract
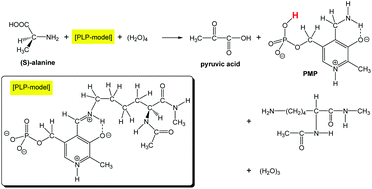
A transamination reaction from an internal aldimine ([PLP]) and (S)-alanine to pyridoxamine phosphate (PMP) and pyruvic acid was investigated by DFT calculations. As [PLP], a model where the lysine (–Lys) part was approximated by –CH[–NH–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH3]–C(
O)–CH3]–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–NH–CH3 was adopted. (H2O)4 was also included to trace reaction paths involving proton transfers. 13 elementary processes were obtained. For (the external aldimine → quinoid), (quinoid → ketimine) and (ketimine → carbinol amine) processes, the water dimer was found to connect a phosphate-group oxygen with the moving proton. The connection promoted the Grotthuss-type proton transfer in transition states. It was revealed that the phosphate group is not a mere substituent but has the central role in the transfer.
O)–NH–CH3 was adopted. (H2O)4 was also included to trace reaction paths involving proton transfers. 13 elementary processes were obtained. For (the external aldimine → quinoid), (quinoid → ketimine) and (ketimine → carbinol amine) processes, the water dimer was found to connect a phosphate-group oxygen with the moving proton. The connection promoted the Grotthuss-type proton transfer in transition states. It was revealed that the phosphate group is not a mere substituent but has the central role in the transfer.


 Please wait while we load your content...
Please wait while we load your content...