Intramolecular axial α/β-coordination of the 132-terminal pyridyl group to the central zinc atom in chlorophyll-a derivatives†
Abstract
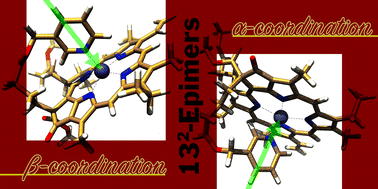
Chlorophyll(Chl)-a derivatives possessing a zinc center and a C132-alkanoate residue with a terminal pyridyl group were synthesized. Their C132-epimerically pure products were isolated by preparative reverse phase HPLC. The C132-stereochemistry resulted in two directed terminal pyridinyl groups that coordinate with the central zinc atom in each stereoisomeric molecule: α/β-intramolecular axial coordination. The asymmetric axial coordinations mimic the immobilization manner of Chl-a in photosynthetically active proteins. The diastereomerically dependent conformers in solution were characterized by 1D/2D NMR, UV-visible absorption, and fluorescence emission as well as circular dichroism (CD) spectroscopy. The 1H NMR and DOSY spectra revealed that the stereoselectively intramolecular coordination occurred in less coordinative deuterated chloroform, whereas a highly coordinative deuterated pyridine molecule replaced the terminal pyridine moiety as the axial ligand to form a mixture of α/β-coordinated species. Their optical spectra in pyridine were nearly independent of the C132-stereochemistry and the linkers in the C132-substituents. The CD bands of β-coordinated species in chloroform were more intense than those of the corresponding α-coordinated stereoisomers, indicating that the former had a larger distortion of the chlorin π-plane than the latter. Therefore, the α-coordinated Chl complex is more conformationally stable than the β-complex.


 Please wait while we load your content...
Please wait while we load your content...