AlCl3-catalyzed regioselective intermolecular α or γ mono- or α,γ bis-hydroalkoxylation of allenamides with alcohols†
Abstract
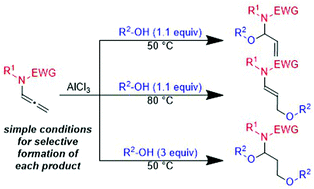
Regioselective intermolecular mono- or bis-hydroalkoxylation of allenamides with alcohols using simple aluminum-catalyzed reaction conditions is reported. When the reaction was carried out with 1.1 equivalents of alcohol at 50 °C, N,O-acetals were generated by 1,2-addition of an alcohol. An increase in temperature to 80 °C leads to γ-substituted ethers by an intermolecular isomerization process. Treatment with an excess of alcohol (3 equiv.) at 50 °C gave 1,3-bis(alkoxy)propanamines. The reactions exhibited good functional group tolerance and efficiency, affording the products in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...