Synthesis of glycopeptides and glycopeptide conjugates
Abstract
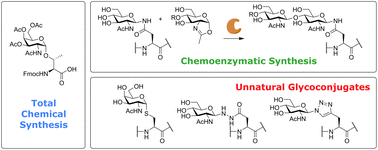
Protein glycosylation is a key post-translational modification important to many facets of biology. Glycosylation can have critical effects on protein conformation, uptake and intracellular routing. In immunology, glycosylation of antigens has been shown to play a role in self/non-self distinction and the effective uptake of antigens. Improperly glycosylated proteins and peptide fragments, for instance those produced by cancerous cells, are also prime candidates for vaccine design. To study these processes, access to peptides bearing well-defined glycans is of critical importance. In this review, the key approaches towards synthetic, well-defined glycopeptides, are described, with a focus on peptides useful for and used in immunological studies. Special attention is given to the glycoconjugation approaches that have been developed in recent years, as these enable rapid synthesis of various (unnatural) glycopeptides, enabling powerful carbohydrate structure/activity studies. These techniques, combined with more traditional total synthesis and chemoenzymatic methods for the production of glycopeptides, should help unravel some of the complexities of glycobiology in the near future.


 Please wait while we load your content...
Please wait while we load your content...