Modulating aptamer function by copper(ii)-mediated base pair formation†
Abstract
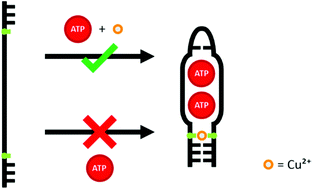
Two aptamers, one for ATP and one for arginine, were modified using an artificial 2′-dexoyribonucleoside based on the nucleobase surrogate imidazole-4-carboxylate. This synthetic nucleoside substitute does not engage in hydrogen bonding but is capable of forming Cu(II)-mediated base pairs instead. Hence, the addition of Cu(II) can be used to influence the ability of the aptamer derivatives to adopt the correct fold necessary for binding their respective target molecule. As a result, aptamer function can be modulated via the addition of Cu(II). The extent of modulation ability depends on the identity of the aptamer and on the exact location of the artificial nucleosides within the oligonucleotide sequence.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...