Regio- and diastereoselective access to densely functionalized ketones via the Boekelheide rearrangement of isoxazoline N-oxides†
Abstract
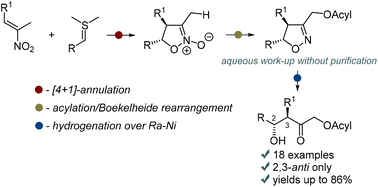
In this work, the classical “isoxazoline route” toward aldols involving the [3 + 2]-cycloaddition of nitrile oxide to alkenes and hydrogenolysis of the oxime group was revisited. To avoid regioselectivity issues, [4 + 1]-annulation of nitroalkenes with sulfonium ylides was used to construct the isoxazoline ring bearing an N-oxide moiety. Subsequent deoxygenative C–H functionalization using the Boekelheide rearrangement and hydrogenolysis of the isoxazoline ring afforded α′-acyloxy-substituted aldols, which are difficult to access both by the classical aldol reaction and the “isoxazoline route”. The products are formed in good to high overall yields and as single diastereomers in most cases. The synthetic use of these aldols was showcased by their smooth transformation into diastereomerically pure triols and a 2,3-diaryl-4-hydroxy-substituted tetrahydrofuran derivative, which is structurally related to cinncassin B.


 Please wait while we load your content...
Please wait while we load your content...