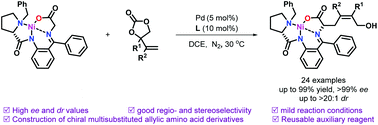
Highly diastereo- and enantioselective synthesis of multisubstituted allylic amino acid derivatives by allylic alkylation of a chiral glycine-based nickel complex and vinylethylene carbonates†
Abstract
The asymmetric synthesis of multisubstituted allylic amino acid derivatives was accomplished by the allylic alkylation of a chiral glycine-based nickel complex with vinylethylene carbonates. High enantioselectivities and diastereoselectivities were obtained under mild reaction conditions. The gram-scale synthesis was carried out with a good yield and high enantioselectivity, indicating that the method is a highly efficient route to chiral multisubstituted allylic amino acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...