A benzothiazole-based dual reaction site fluorescent probe for the selective detection of hydrazine in water and live cells†
Abstract
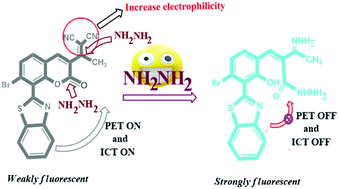
As hydrazine is an environmental pollutant and highly toxic to living organisms, selective and rapid detection is highly needed for the benefit of living organisms as well as the environment. Here, we first introduced a novel benzothiazole conjugated methyldicyanovinyl coumarin probe BTC, with dual recognition sites for hydrazine detection. The incorporation of the methyldicyanovinyl group into the benzocoumarin fluorophore increased the electrophilicity of the lactone ring of the probe BTC facilitating the nucleophilic attack of hydrazine and rapid (within 1 min, low detection limit = 1.7 nM) turn-on sky blue fluorescence with 700-fold fluorescence intensity enhancement was observed via hydrazine-induced lactone ring-opening followed by selective cleavage of the dicyanovinyl group. According to the literature, dicyanovinyl group assisted lactone ring opening has revealed the possibility of hydrazine recognition with a large Stokes shift (140 nm) and a high fluorescence quantum yield (0.67). Here, the DFT study and practical applications of the probe BTC in different water samples have been presented. The probe BTC was also successfully applied for the detection of hydrazine in the vapor phase using paper strips and in live MDA-MB 231 cells.


 Please wait while we load your content...
Please wait while we load your content...