Photoinduced radical cascade cyclization of acetylenic acid esters with oxime esters to access cyanalkylated coumarins†
Abstract
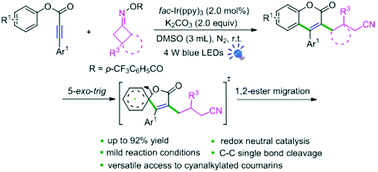
A photoinduced radical cascade cyclization of acetylenic acid esters with oxime esters is described, providing cyanalkylated coumarins in superior yields under mild conditions. Radical capture and luminescence quenching experiments showed that this transformation was accomplished via a radical addition/5-exo spirocyclization/1,2-ester migration process.


 Please wait while we load your content...
Please wait while we load your content...