Heteroaryl sulfonamide synthesis: scope and limitations†
Abstract
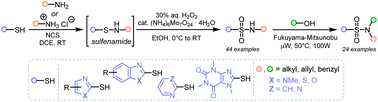
Heteroaryl sulfonamides are important structural motifs in the medicinal and agrochemical industries. However, their synthesis often relies on the use of heteroaryl sulfonyl chlorides, which are unstable and toxic reagents. Herein, we report a protocol that allows direct oxidative coupling of heteroaryl thiols and primary amines, readily available and inexpensive commodity chemicals. The transformation proceeds under mild reaction conditions and yields the desired N-alkylated sulfonamides in good yields. N-alkyl heteroaryl sulfonamides can be further transformed using a microwave-promoted Fukuyama–Mitsunobu reaction to N,N-dialkyl heteroaryl sulfonamides. The developed protocols thus enable the preparation of previously difficult to prepare sulfonamides (toxic reagents, harsh conditions, and low yields) under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...