A micro-flow rapid dual activation approach for urethane-protected α-amino acid N-carboxyanhydride synthesis†
Abstract
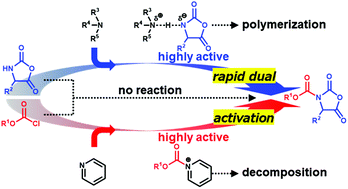
This study demonstrated the rapid dual activation (10 s, 20 °C) of a combination of an α-amino acid N-carboxyanhydride and alkyl chloroformate in the synthesis of a urethane-protected α-amino acid N-carboxyanhydride in a micro-flow reactor. The key to success was the combined use of two amines that activated both substrates with proper timing. Three amines, i-Pr2NEt, Me2NBn, or N-ethylmorpholine, were used with pyridine in accordance with the steric bulkiness of a side chain in the α-amino acid N-carboxyanhydride. A variety of 16 urethane-protected α-amino acid N-carboxyanhydrides were synthesized in high yields. The role of amines was investigated based on the measurement of the time-dependent (0.5 to 10 s) decrease of α-amino acid N-carboxyanhydrides and alkyl chloroformates in the presence of amines via flash mixing technology using a micro-flow reactor. It was suggested that the in situ generated acylpyridinium cation was highly active and less prone to causing undesired decomposition compared with the acylammonium cation examined in this study. Thus, even at a very low concentration, the acylpyridinium cation facilitated the desired coupling reaction.


 Please wait while we load your content...
Please wait while we load your content...