ZnCl2-mediated stereo- and chemoselective synthesis of vinylphosphonates†
Abstract
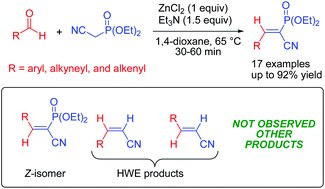
A highly chemo- and stereoselective synthesis of diethyl (E)-2-(alkylidene)-2-phosphonoacetonitriles via the Knoevenagel condensation reaction of carbonyl compounds with diethyl cyanomethylphosphonate in the presence of zinc chloride has been achieved. By the presented method, various E-isomers of arylmethylidene phosphonates rather than Horner–Wadsworth–Emmons olefination products were obtained in good to excellent yields. Their E configurations were determined by X-ray diffraction and NMR analyses. In addition, DFT calculations provided insights into the chemo- and stereoselectivity of the reaction.

 Please wait while we load your content...
Please wait while we load your content...