Transition-metal-catalyzed one-pot selenylation of electrophilic arylating agents using triphenyltin chloride/Se as a phenylselenating agent†
Abstract
Three novel and efficient protocols for the synthesis of phenyl aryl selenides through a three-component coupling reaction of triphenyltin chloride with aryl halides, phenolic esters or nitroarenes, and Se powder catalyzed by CuI or Cu(OAc)2 in the presence of a base in PEG200 at 90–100 °C have been developed. Also, NiFe2O4 as a magnetically reusable nanocatalyst was applied in these reactions under similar reaction conditions. The present methods are superior to other currently available methods due to the use of triphenyltin chloride/Se as a phenylselenating agent and phenolic esters and nitroarenes as a coupling partner for C–Se–C bond formation for the first time, a green solvent and inexpensive and reusable catalysts, and avoidance of any toxic and expensive arylselenating reagents.
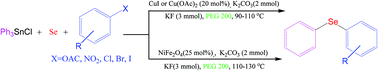


 Please wait while we load your content...
Please wait while we load your content...