Convergent access to mono-fluoroalkene-based peptidomimetics†
Abstract
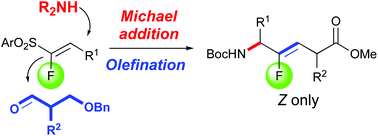
The convergent and selective preparation of (Z)-monofluoroalkene-based dipeptide isosteres from functionalized fluorosulfones as a cornerstone is described. In this approach, the N-terminal amino group is introduced by a conjugate addition reaction of phthalimide onto fluorinated vinylsulfones containing α-amino-acid side chains while the C-terminal motif is linked to the fluorovinylic peptide bond mimic via the Julia–Kocienski reaction between fluorosulfones and substituted aldehydes bearing α-amino-acid side chains.


 Please wait while we load your content...
Please wait while we load your content...