Hydroboration and reductive amination of ketones and aldehydes with HBpin by a bench stable Pd(ii)-catalyst†
Abstract
A palladium(II) complex [(κ4-{1,2-C6H4(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C6H4O)2}Pd] (1) supported by a dianionic salen ligand [1,2-C6H4(N
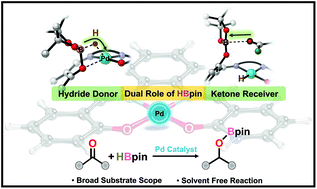
CH–C6H4O)2}Pd] (1) supported by a dianionic salen ligand [1,2-C6H4(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C6H4O)2]2− (L) was synthesised and used as a molecular pre-catalyst in the hydroboration of aldehydes and ketones. The molecular structure of Pd(II) complex 1 was established by single-crystal X-ray diffraction analysis. Complex 1 was tested as a competent pre-catalyst in the hydroboration of aldehydes and ketones with pinacolborane (HBpin) to produce corresponding boronate esters in excellent yields at ambient temperature under solvent-free conditions. Further, the complex 1 proved to be a competent catalyst in the reductive amination of aldehydes with HBpin and primary amines under mild and solvent-free conditions to afford a high yield (up to 97%) of corresponding secondary amines. Both protocols provided high conversion, superior selectivity and broad substrate scope, from electron-withdrawing to electron-donating and heterocyclic substitutions. A computational study based on density functional theory (DFT) revealed a reaction mechanism for Pd-catalysed hydroboration of carbonyl species in the presence of HBpin. The protocols also uncovered the dual role of HBpin in achieving the hydroboration reaction.
CH–C6H4O)2]2− (L) was synthesised and used as a molecular pre-catalyst in the hydroboration of aldehydes and ketones. The molecular structure of Pd(II) complex 1 was established by single-crystal X-ray diffraction analysis. Complex 1 was tested as a competent pre-catalyst in the hydroboration of aldehydes and ketones with pinacolborane (HBpin) to produce corresponding boronate esters in excellent yields at ambient temperature under solvent-free conditions. Further, the complex 1 proved to be a competent catalyst in the reductive amination of aldehydes with HBpin and primary amines under mild and solvent-free conditions to afford a high yield (up to 97%) of corresponding secondary amines. Both protocols provided high conversion, superior selectivity and broad substrate scope, from electron-withdrawing to electron-donating and heterocyclic substitutions. A computational study based on density functional theory (DFT) revealed a reaction mechanism for Pd-catalysed hydroboration of carbonyl species in the presence of HBpin. The protocols also uncovered the dual role of HBpin in achieving the hydroboration reaction.


 Please wait while we load your content...
Please wait while we load your content...