Pd and photoredox dual catalysis assisted decarboxylative ortho-benzoylation of N-phenyl-7-azaindoles†
Abstract
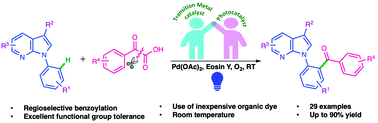
The directing group assisted decarboxylative ortho-benzoylation of N-aryl-7-azaindoles with α-keto acids has been achieved by synergistic visible light promoted photoredox and palladium catalysis. The approach tenders rapid entry to aryl ketone architectures from simple α-keto acid precursors via the in situ generation of a benzoyl radical intermediate. The transformation provides a range of ortho-benzoylated N-aryl-7-azaindoles, with excellent site-selectivity and good functional group compatibility under mild reaction conditions. Biological target predictions indicate that these molecules may serve as potential anti-cancer and anti-viral agents.


 Please wait while we load your content...
Please wait while we load your content...