Effect of a cis-4-aminopiperidine-3-carboxylic acid (cis-APiC) residue on mixed-helical folding of unnatural peptides†
Abstract
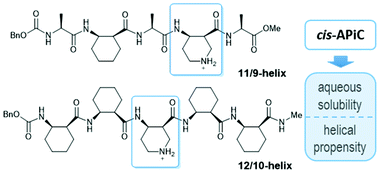
The α/β-peptide 11/9-helix and the β-peptide 12/10-helix belong to “mixed” helices, in which two types of hydrogen bonds with opposite directionality alternate along the helical axis. cis-2-Aminocyclohexanecarboxylic acid (cis-ACHC) is known to promote these mixed helices and stabilize the helical propensity more than other acyclic β-residues. Application of a mixed-helical backbone still requires sufficient solubility in aqueous solution. In this regard, we chose cis-4-aminopiperidine-3-carboxylic acid (cis-APiC) as a foldamer building block that can provide both sufficient aqueous solubility and mixed-helical propensity. Conformational analyses of α/β- and β-peptides containing a cis-APiC residue by circular dichroism spectroscopy and single-crystal X-ray crystallography suggest that the incorporation of cis-APiC instead of cis-ACHC can enhance the aqueous solubility of the mixed-helical peptides without any adverse effect on helical folding. In addition, the ratio between right- and left-handed 12/10-helices of β-peptides can be rationalized by relative energies between the local conformations of the cis-APiC residue.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...