A flow-based transition-metal-catalysed hydrogenolysis strategy to facilitate peptide side-chain deprotection†
Abstract
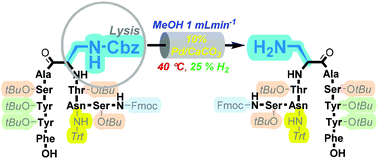
Orthogonal deprotection methodologies are an invaluable tool for the construction of site-specially modified peptides. Here, we report a facile 10% Pd/CaCO3-based procedure to selectively mediate Nβ-side-chain Cbz-lysis from extended peptide sequences in the presence of trityl and t-Butyl protecting groups.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...