Construction of aziridine, azetidine, indole and quinoline-like heterocycles via Pd-mediated C–H activation/annulation strategies
Abstract
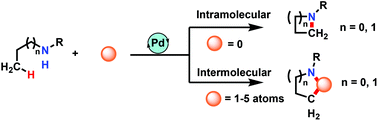
N-Heterocycles can be found in natural products and drug molecules and are indispensable components in the area of organic synthesis, medicinal chemistry and materials science. The construction of these N-containing heterocycles by traditional methods usually requires the preparation of reactive intermediates. In the past decades, with the rapid growth of transition metal catalysed coupling reactions, syntheses of heterocycles from precursors with inert chemical bonds have become a challenge. More recently, in the field of transition metal associated C–H direct functionalization, efficient methods have been developed for the syntheses of N-heterocyclic compounds such as aziridines, azetidines, indoles and quinolines under the click type of reaction mode. In this review, representative synthetic methodologies developed in the recent 10 years for the preparation of this small class of N-heterocycles via the Pd-catalysed C–H activation and C–N bond formation pathway are discussed. We hope this article will provide new insights from the strategies highlighted into future molecular design, synthesis and applications in medical and materials sciences.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...